AskGene Pharma Dosed First Patient in Phase 3, Pivotal Trial of ASKB589 in First-Line Advanced Gastric and Gastroesophageal Junction Cancers
Camarillo, California, January 26, 2024 – AskGene Pharma Inc. announces dosing of the first patient at a participating site in China for the Phase III pivotal trial of ASKB589. ASKB589 is a CLDN18.2 targeting antibody that is being tested in combination with CAPOX and a PD-1 inhibitor. This combination therapy is intended for the first-line treatment patients with advanced, recurrent, or metastatic gastric cancer (GC) and gastroesophageal junction (GEJ) cancer in China. This study marks the first pivotal clinical trial for triple combination therapy using an anti-claudin 18.2 antibody alongside a PD-1 inhibitor and chemotherapy, for the initial treatment of G/GEJ cancer patients (NCT04632108).
Jian-Feng (Jeff) Lu, Ph.D., CEO of AskGene, commented: “We are very happy that our pivotal phase 3 program has successfully started in China with the enrollment of the first patient. The promising results of Phase 1/2 study of triple combination suggest that the combination of ASKB589, PD-1 inhibitor, and chemotherapy may be a transformative treatment option for gastric or gastroesophageal junction adenocarcinoma and support exploring the combination in patients with earlier stages of disease. The triple combination represents an innovative approach which aims to leverage the full potential of targeted therapy, immunotherapy and chemotherapies.”
The multicenter, randomized, double-blind, standard-of-care controlled Phase 3 trial (NCT06206733) has a planned enrollment of 780 patients. Patients will receive ASKB589 plus tislelizumab and CAPOX or placebo plus tislelizumab and CAPOX on day 1 of each 3-week cycle. The primary endpoint of the trial is progression free survival (PFS), with overall survival (OS), objective response rate (ORR), duration of response (DOR) and safety serving as secondary endpoints.
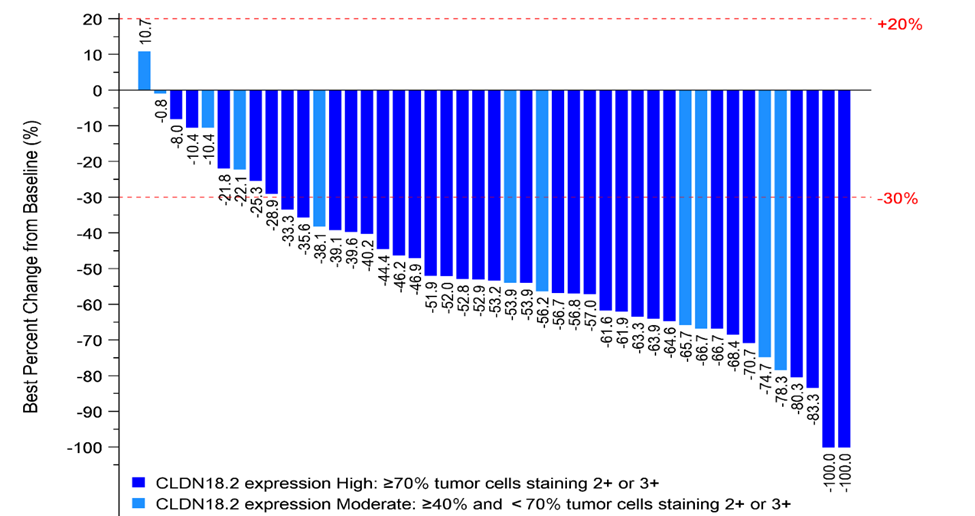
The latest Phase I/II clinical results presented at ASCO GI (the American Society of Clinical Oncology Gastrointestinal Cancer Symposium) in January 2024 demonstrate an encouraging anti-tumor activity and a manageable safety profile for ASKB589 combination therapy with CAPOX and a PD-1 inhibitor1. Among 45 CLDN18.2 moderate to high patients (determined by a validated proprietary companion diagnostic kit) with measurable disease and at least one post-treatment tumor assessment in the 6 mg/kg dose-expansion phase, 36 (80.0%) patients achieved Partial Response (PR) and 9 (20.0%) achieved Stable Disease as the best overall tumor response per RECISTv1.1. Disease Control Rate (DCR) was 100%.
About ASKG589
ASKB589 is a second generation anti-CLDN18.2 humanized monoclonal antibody with ADCC enhancement. To support the Phase 3 pivotal trial, more than 200 patients have been enrolled into 2 Phase I/II studies, including ASKB589 monotherapy, combination with chemotherapy, and combination with PD-1 inhibitor and chemotherapy. No dose limiting toxicity (DLT) has been observed so far, with monotherapy up to 20 mg/kg, and combination therapy up to 15 mg/kg. A maximum tolerated dose (MTD) has not yet been reached. ASKB589 is intended for treatment of G/GEJ cancer, pancreatic cancer, and additional cancer types which express CLDN18.2.
AskGene has also developed a sensitive and specific CLDN18.2 immunohistochemistry-based companion diagnostic kit, which is used to support ASKB589 Phase III program.
About AskGene Pharma
AskGene Pharma, founded in 2012 in Camarillo, California, is dedicated to the discovery and development of novel antibodies and fusion protein therapeutics. It has established the proprietary SmartKine® cytokine prodrug platform, which significantly improves the developability of cytokines for oncology and inflammation indications. AskGene has multiple programs in clinical and preclinical developments. In addition to the cytokine programs, AskGene is also developing ASKB589, an anti-CLDN18.2 monoclonal antibody currently in Phase 3 for G/GEJ cancer, and ASKG712, a bifunctional molecule targeting both VGEF and ANG-2 pathways currently in phase 1 development for ophthalmology indications.
Forward-looking statement Disclaimer
This press release contains forward-looking statements that involve risks, uncertainties, and assumptions. These forward-looking statements reflect the Company’s views at the time such statements were made with respect to future events and are not a guarantee of future performance or developments. All statements other than statements of historical fact may be deemed as forward-looking, including but not limited to: any statements regarding plans, strategies and objectives for the future management of operations, including but not limited to, our clinical development and commercialization plans; predictions of any financial information; any statement of historical results that may indicate trends in our business development; statements of any expectations or beliefs regarding future events, potential markets or market size, technological developments, product lines, clinical data, results, experiments or their implications, enforceability of intellectual property rights, competitive advantage or our position in the industry; and any hypothetical statements of the items mentioned.
Reference:
1Peng Z, Shen L, He Y, et. Al. A phase Ib/II study of ASKB589 (anti-Claudin 18.2 [CLDN18.2] monoclonal antibody) combined with CAPOX and PD-1 inhibitor as first-line treatment for locally advanced, relapsed and metastatic gastric/gastro-esophageal junction (G/GEJ) adenocarcinoma. ASCO GI 2024, Abstract # 317, Poster # E17.