AskGene Presents Interim Results of ASKB589 (anti-CLDN18.2 antibody) in Combination with CAPOX and PD-1 Inhibitor at ASCO-GI 2024
Camarillo, California, January 18, 2024 – AskGene Pharma Inc. presented encouraging clinical results for ASKB589, an anti-CLDN18.2 antibody, at the American Society of Clinical Oncology Gastrointestinal Cancer Symposium (ASCO-GI 2024) in San Francisco on January 18-20, 2024.
The results were from clinical trial NCT05632939, a Phase Ib/II, two-part, dose escalation and expansion study to evaluate the safety, tolerability, and anti-tumor activities of ASKB589 in combination with chemotherapy (CAPOX) and a PD-1 inhibitor as a first-Line treatment in patients with locally advanced, relapsed and metastatic gastric (G) or gastroesophageal junction (GEJ) adenocarcinoma. As of December 20, 2023, a total of 62 patients with positive CLDN18.2 expression were dosed Q3W with ASKB589 combined with CAPOX and PD-1 inhibitor: 9 patients received ASKB589 at 6 mg/kg (n=3) and 10 mg/kg (n=6) in the dose escalation and 53 patients at 6 mg/kg in the dose expansion. Most of the enrolled patients had moderate to high CLDN18.2 expression (83.9%) and PD-L1 CPS ³1 (59.7%).
ASKB589 was safe and tolerated at both 6 and 10 mg/kg. No dose-limiting toxicity was observed, and maximum tolerated dose (MTD) was not identified during the escalation phase of the study. Most adverse events (AEs) were of mild severity (grade 1 or 2). The most common AEs were hypoalbuminemia (77.4%), nausea (66.1%), anemia (56.5%), neutrophil count decreased (54.8%), and vomiting (51.6%). No patients discontinued the treatment due to AEs.
Among 45 CLDN18.2 moderate to high patients (determined by a validated proprietary companion diagnostic kit) with measurable disease and at least one post-treatment tumor assessment in the 6 mg/kg dose- expansion phase, 36 (80.0%) patients achieved partial response and 9 (20.0%) achieved stable disease as the best overall tumor response per RECISTv1.1. Disease control rate was 100%. As of the cutoff date, 41(77.3%) out of the 53 patients in the dose expansion group were still on treatment.
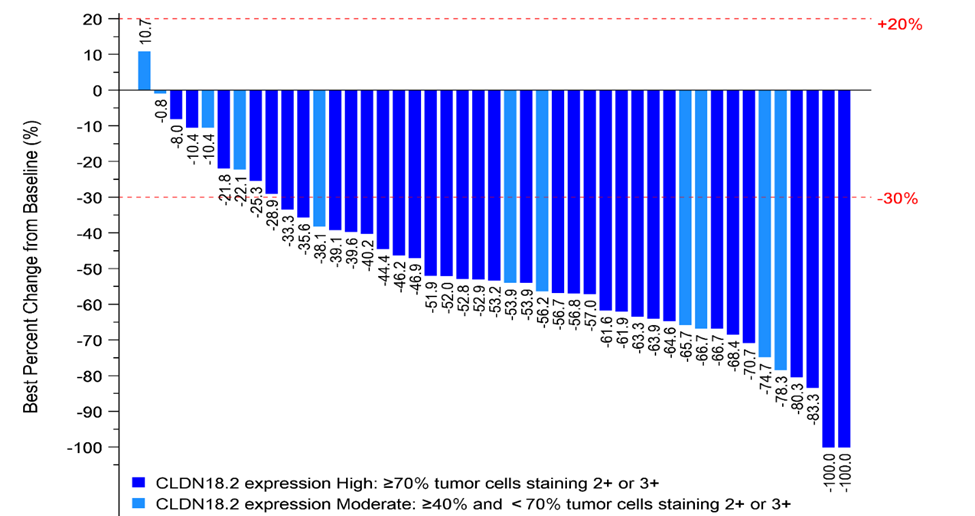
In summary, ASKB589 plus CAPOX and PD-1 inhibitor as a first-line treatment in patients with G/GEJ cancer demonstrated good safety and tolerability. Adding a PD-1 inhibitor to ASKB589 plus CAPOX in patients with moderate to high CLDN18.2 expression resulted in encouraging anti-tumor activities with deep and durable responses. Based on the interim results of this study, NMPA greenlighted the Phase 3 study of ASKB589 in combination with CAPOX and PD-1 inhibitor as a first-line treatment in CLDN18.2 moderate to high (≥40% 2+/3+ staining) patients with advanced G/GEJ cancer in China.
Jian-Feng (Jeff) Lu, Ph.D., CEO of AskGene, commented: “Claudin 18.2 has recently been validated as a new molecular target that demonstrates clinical benefit for patients with gastric and gastroesophageal cancer. Our study shows that triple combination of ASKB589, chemotherapy, and PD-1 inhibitor can be administered safely in patients and results in a very high rate of deep and durable responses, as well as a 100% disease control rate. We have initiated the registrational trial and expect to enroll the first patient soon”.
Presentation Details
- Title: A Phase Ib/II Study of ASKB589 (Anti-CLDN18.2 Monoclonal Antibody) in Combination with CAPOX and PD-1 Inhibitor as a First-Line Treatment of Locally Advanced, Relapsed and Metastatic G/GEJ Cancer (NCT05632939)
- Principle Investigator: Dr. Lin Shen, Peking University Cancer Hospital
- Presenter: Dr. Zhi Peng, Peking University Cancer Hospital
- Abstract #: 317
- Poster #: E17
About NCT05632939
The NCT04632108 study is a Phase Ib/II, two-part, dose escalation and expansion study to evaluate the safety, tolerability, and anti-tumor activities of ASKB589 in combination with chemotherapy (CAPOX) and a PD-1 inhibitor as a first-Line treatment in patients with locally advanced, relapsed and metastatic G/GEJ cancer. The study includes ASKB589 dose escalation (6 and 10 mg/kg) and expansion study of ASKB589 (6 mg/kg) combined with CAPOX and PD-1 inhibitor. Patients with positive CLDN18.2 expression (any tumor cell with ≥1+ membrane staining) determined by the central lab have been enrolled.
About ASKB589
ASKB589 is an innovative biological drug discovered and developed by AskGene. It is a recombinant humanized monoclonal antibody targeting CLDN 18.2. The drug mediates antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) through high-affinity binding to CLDN18.2-expressing cancer cells. ASKB589 is intended for treatment of G/GEJ cancer, pancreatic cancer, and additional cancer types which express CLDN18.2.
About AskGene Pharma
AskGene Pharma, founded in 2012 in Los Angeles, California, is dedicated to the discovery and development of novel antibodies and fusion protein therapeutics. It has established the proprietary SmartKine® cytokine prodrug platform, which significantly improves the developability of cytokines for oncology and inflammation indications. AskGene has multiple programs in clinical and preclinical developments. In addition to the cytokine programs, AskGene is also developing ASKB589, an anti-CLDN18.2 monoclonal antibody currently in Phase 3 for G/GEJ cancer, and ASKG712, a bifunctional molecule targeting both VGEF and ANG-2 pathways currently in phase 1 development for ophthalmology indications.